International Codes of Practice on equine disease for 2023
Article by Victoria Colgate and Richard Newton
Horses are one of the most internationally travelled species, second only after humans, and this mobility, both between and within countries, means that the spread of equine infectious diseases is a very real and ever-present threat.
Indeed, infectious disease outbreaks are often related to new arrivals at, or movement of animals on and off premises, such as to competitions and race meetings. As well as having a negative impact on horse health and welfare, disease outbreaks can have further reaching consequences in terms of treatment costs, economic losses due to movement restrictions and an inability to compete, as well as disruption to the breeding schedule, which may have effects in racing in future years. Additionally, restrictions imposed in the face of the diagnosis of certain diseases can prevent the free export of horses between countries, impacting trade and equestrian sport. Infectious diseases are truly trans-boundary, and such a problem necessitates global cooperation and communication, echoing the mantra that ‘prevention is better than cure’.
The origins and evolution of the Codes of Practice
Figure 1
In 1977 in Newmarket, UK, there was widespread development of vulval discharge in thoroughbred mares post-covering that adversely affected their fertility and to an extent that caused temporary closure of stallion barns and stud farms. Initially unknown, the cause of this outbreak was later identified as the bacterium Tayorella equigenitalis, the causative agent of contagious equine metritis (CEM) and often referred to as the CEM organism, or CEMO.
The impact on the 1977 breeding season was significant enough for the Horserace Betting Levy Board (HBLB) to consider a serious need to control CEM in future years and in reaction to this, the Codes of Practice (CoP) were created. First developed by HBLB committee discussion in the summer of 1977 and then formally published in 1978, the HBLB CoP outlined swabbing protocols in the weeks prior to covering in an attempt to prevent the venereal transmission of CEM.
Despite the perceived draconian nature of these first codes, compliance was high and overall were highly successful—with CEM cases drastically falling following their introduction. In subsequent years, the CEM CoP was extended to include control measures on reproductive disease caused by the other venereal bacterial pathogens: Klebsiella pneumoniae (capsule types 1, 2 and 5) and Pseudomonas aeruginosa. Codes on the prevention and management of equine herpesvirus-1 (EHV-1) and equine viral arteritis (EVA) were also added following disease outbreaks of significant concern to the thoroughbred breeding industry in subsequent years.
Today the CoP are referred to as the ‘HBLB International Codes of Practice’, with the 2020 CoP being the inaugural internationally branded edition and representing a comprehensive manual outlining a series of voluntary standards (codes) and advisories (guidelines), with accompanying appendices. They are intended to assist breeders, trainers and horse owners (in collaboration with their veterinary surgeons) to control and prevent a range of important infectious diseases in equids. The CoP have a broad application among thoroughbred breeders; and the UK, Ireland, France, Germany and Italy are all signatories. Additionally, in a translated form, they have formed the basis of written equine infectious disease advice in many other countries. The initial reduction and then sustained maintenance of low levels of infectious disease outbreaks experienced after the CoP’s introduction is testament to the document’s effectiveness and importance.
The HBLB International Codes of Practice for the 2023 breeding season
The CoP are reviewed annually by a group of international veterinary breeding and infectious disease experts and stakeholder representatives. This review ensures that all advice is as current as possible regarding the latest scientific evidence and global disease situation. The CoP convey practical recommendations gained considering recent experiences with the occurrence and control of relevant outbreaks.
The 2023 edition of ‘The CoP Manual’ (title cover, above Fig 1 and table of contents, below Fig 2) comprises:
Figure 2
Six Codes on the following diseases: CEM (covering CEMO, Klebsiella pneumoniae and Pseudomonas aeruginosa), EVA, EHV-1, equine coital exanthema (ECE, caused by EHV-3), equine infectious anaemia (EIA) and dourine
Five Guidelines on equine influenza (EI), piroplasmosis, strangles, West Nile Fever (WNF) and artificial insemination (AI)
Eleven Appendices with a range of supporting information and guidance
The CoP are published on the HBLB website (https://codes.hblb.org.uk/); a PDF download (https://codes.hblb.org.uk/downloads/2023/Codes%20of%20Practice%202023.pdf) is also available. In Great Britain, the Thoroughbred Breeders Association (TBA) produces a spiral-bound printed hard copy for its members.
The CoP outline each disease sequentially, using a standardised format of sections which include information on notification procedures, clinical signs, transmission, prevention, diagnosis, control, treatment, freedom from disease and export requirements. It is a document that continues to evolve over time in terms of both the diseases included and the expert advice imparted.
Why ‘codes’ and ‘guidelines’?
Although the logic behind the distinction as to why specific diseases in the CoP are covered by a guideline rather than a code is not necessarily immediately initiative. It is worth remembering that a Code of Practice may be defined as ‘A documented set of recommended or preferred processes, actions or organisational structures to be applied in a given setting’, whereas a guideline is: ‘A general rule, principle or piece of advice’. Therefore, the diseases applied as codes are those that directly relate to, and have an impact on, breeding and that necessitate particular actions either to prevent or control disease, should they occur. The guidelines, in contrast, are merely advisory measures to those involved in thoroughbred breeding businesses, but cover diseases and practices that are also highly applicable to other populations of horses. The remainder of the article outlines several of the important codes and guidelines.
Strangles
Strangles, caused by the bacterium Streptococcus equi, is a disease affecting the lymph nodes of the upper respiratory tract. Although endemic within the UK horse population, it only occasionally affects thoroughbred stud farms. Accordingly, it was first included in the CoP as an advisory guideline in 2004 and has since been periodically updated in line with developments in diagnostic testing and vaccination. Due to the ability of Streptococcus equi to develop persistent infection, remaining within material (pus/chondroids) in the guttural pouches long after resolution of clinical signs, one of the key elements in prevention and control is identification and treatment of these carrier animals. This involves performing guttural pouch endoscopy and lavage of strangles cases around 30 days after clinical recovery from infection, and also of any new arrivals at a premises during the quarantine period, before they are allowed to mix with the resident herd.
Equine infectious anaemia (EIA)
Figure 3
EIA, also known as swamp fever, is caused by the equine infectious anaemia virus (EIAV) and is transmitted between horses by the transfer of infected blood, either by insect vectors or contaminated veterinary equipment or through administration of infected blood products (e.g., plasma or whole blood transfusion). It is found in thoroughbred and non-thoroughbred populations worldwide, including parts of mainland Europe; but it is currently not present in Great Britain, where any suspicion of disease is notifiable by law to the Animal and Plant Health Agency (APHA) and would result in compulsory slaughter of the affected animal. Following an outbreak in Ireland in 2006, a CoP for EIA was developed by Defra in the UK in August that year and was included as an addendum for the 2007 breeding season (Figure 3) and subsequently as a full code from 2008 onwards. EIA has been included as a full CoP since 2008, and it recommends pre-breeding testing of all mares and stallions prior to commencement of the covering season as the best way to establish and maintain freedom from infection.
Dourine
A new code on dourine was added in 2012, following its re-identification in Sicily and the Italian mainland during 2011, which necessitated pre-import screening of horses arriving from this area. A notifiable venereal disease caused by the protozoan parasite Trypansoma equiperdum, once widespread, had largely undergone eradication and of late had only been reported in Asia, Africa, South America, Eastern Europe, Mexico and Russia. There is no cure for dourine, and euthanasia is usually the advised treatment option on the grounds of animal welfare and population health. As investigations into the 2011 Italian outbreak indicated subclinical seropositivity to dourine in many regions of Italy, it was evident that the disease was closer to our shores than anticipated. That led to its addition to the CoP in order to keep all owners/breeders informed and appropriately advised.
Equine influenza (EI)
EI was added to the CoP in 2020 as an advisory guideline following the 2019 European epidemic, which saw a major outbreak in the UK (Figure 4), including cases of clinical disease in vaccinated thoroughbreds. This led to the cancellation of British horseracing for six days in February 2019 as a pre-emptive control measure, but still at significant economic cost to the industry.
Figure 4
Although clinical EI is usually fairly mild and self-limiting, the resulting damage to the respiratory epithelium can impact performance for up to six months and leaves the horse vulnerable to secondary bacterial infections and potential pneumonia. Appropriate vaccination, biosecurity protocols and effective quarantining of new arrivals are outlined in the Code as the cornerstone to EI control. With such a highly contagious virus capable of spreading over large distances and with great speed, especially in the immunologically naïve, awareness and prevention are key.
West Nile fever (WNF)
WNF, caused by West Nile virus (WNV), is an infectious but non-contagious disease transmitted through the bite of an infected mosquito. Although the natural host-vector cycle is between birds and mosquitoes, horses and indeed humans can become infected but act as so-called incidental or ‘dead-end’ hosts; importantly, not presenting is a risk for onward transmission as there is insufficient WNV in their bloodstream. Many horses infected with WNV remain without signs, but approximately 10% will develop neurological disease, which can be fatal. Historically confined to Africa and the East, it entered North America in 1999 leading to widespread infection with many equine and human fatalities. Since then it has become endemic in the USA and continues to spread further into northern Europe as climate change alters vector habitats and life cycles. After the development of equine cases in Germany in 2018 and evidence of human and bird cases in the Netherlands in 2020, WNV was considered to pose an increasing threat to the UK horse population, especially animals that travelled overseas for competition and breeding purposes. WNF was therefore added as a guideline to the CoP in 2021.
Piroplasmosis
The latest disease addition to the CoP was an advisory guideline on piroplasmosis in 2022, following concern that the disease was becoming increasingly important among the international equine population. Piroplasmosis is a tick-borne disease caused by the intracellular parasites Babesia caballi and Theileria equi. Although the UK is currently considered to be free from locally acquired endemic disease (referred to as ‘autochthonous’), cases have occasionally been confirmed in the UK and are endemic in other European countries. With no formal requirements for pre-import screening, infection could re-enter the UK through importation of infected horses. The code gives informative background information to raise awareness among thoroughbred breeders and owners/keepers of other horse populations.
Improving accessibility and applicability of the CoP
Another way in which the CoP have more recently evolved is through the mechanisms of delivery to stakeholders. In July 2016, the accessibility and reach of the CoP took a further leap with the generation of the smartphone EquiBioSafe app (https://play.google.com/store/apps/details?id=com.veterinaryadvances.android.equibiosafe&hl=en_GB&gl=US&pli=1). With the HBLB Codes of Practice and National Trainers Federation Codes of Practice précised into key elements for the control and prevention of infectious diseases, the app allows interactive and stable-side access to advice, as well as assisting trainers to comply with sporting authority vaccine regulations and disease notification procedures. With the ability to send emergency notifications in the event of heightened disease threats in a particular area, the app provides real-time relevant information to assist with implementation of proactive biosecurity measures. This helps safeguard horse health and the socioeconomic livelihoods of all those involved in equestrian sport. Like the CoP, the EquiBiosafe app has mainly been targeted to a European audience, but downloads recorded from North America, Asia and Latin America demonstrate its international application.
The HBLB International Codes of Practice act as broad, minimum requirement recommendations for the identification, treatment, prevention and control of a range of important equine infectious diseases—equally relevant across international borders and from pleasure to elite competition horses. They are also dynamic, evolving over time in line with the ever-changing disease situation, and therefore acting as a vital education and reference resource to all those involved in the equestrian industry. They form a user-friendly instruction manual of exactly ‘how (not) to’ allow infectious diseases to fulfil their devastating potential.
How Equine Influenza viruses mutate
By Debra Elton and Adam Rash
Overview
Equine influenza virus (EIV) causes equine influenza in horses, characterised by a raised temperature and harsh dry cough and rapid transmission amongst unprotected horses. It is a major threat to the thoroughbred racing industry as it has the potential to spread so quickly and can cause the cancellation of events and restriction of horse movement. The last major outbreak in Europe occurred in 2003, when over 1000 vaccinated horses in Newmarket became infected. The virus spread throughout the UK and outbreaks were also reported in Ireland and Italy. More recently, more than 50,000 horses were infected during the 2007 outbreak in Australia, large-scale outbreaks occurred in India during 2008 and 2009 and multiple countries were affected by widespread outbreaks in South America in 2012. At the time of writing, another widespread outbreak has been affecting South America, with reports from Chile, Argentina, Uruguay and Colombia to date. International transport of horses for events and breeding purposes means that equine influenza can spread readily from one country to another. Infected horses can shed the virus before they show any clinical signs of infection and vaccinated animals can be infectious without showing any obvious signs, adding to the risk.
Regular vaccination against equine influenza offers the best protection against infection. Three major vaccine manufacturers make products for the European market, each differing in the virus strains that are included in the vaccine. Sophisticated adjuvants are included in these vaccines, which help boost the horse’s immune response. However, EIV, like other influenza viruses, can mutate to change its surface proteins and can thereby escape from immunity generated by vaccination. It is important that vaccines contain relevant vaccine strains, to give them the best chance of working against current EIVs.
EIV belongs to the influenza A group of viruses, which infect a variety of other animals including humans, birds, pigs and dogs. The natural reservoir for most influenza A viruses is wild aquatic birds, from this pool some viruses go on to infect new hosts and adapt to spread in them. Influenza A viruses are subtyped according to two proteins found on the surface of the virus, haemagglutinin (HA) and neuraminidase (NA). Sixteen HA subtypes and 9 NA subtypes are found in aquatic birds, however only two subtypes are known to have become adapted to horses, H3N8 and H7N7. Equine H7N7 viruses were first isolated in 1956 but have not been isolated since the late 1970s and are now thought to be extinct. Equine H3N8 viruses were first isolated in 1963 when they caused an influenza pandemic in horses and continue to circulate today.
Antigenic drift and shift
International travel of horses means the virus can spread readily from one country to another.
The HA and NA proteins on the surface of the influenza virus particle induce antibodies in the host when the virus infects it. For EIV, these antibodies protect the horse against further infection provided the horse encounters similar viruses. A similar process occurs when horses are immunised with a vaccine, most vaccines contain virus proteins that induce the horse’s immune system to make protective antibodies. However, the response to the vaccine is not as good as to virus infection, so horses need to be vaccinated regularly to maintain a protective immune response.
To overcome the horse’s immune response and enable the virus to survive in the equine population, EIV gradually makes changes to its surface proteins. This process is called antigenic drift. The result is that eventually the horse’s antibodies no longer recognise the virus, which is then able to infect the animal. The two proteins that are important for antigenic drift are HA and NA. HA is involved in virus entry into target cells of the respiratory tract. Antibodies against HA block virus infection, either by preventing the virus from binding to the cell surface, or by preventing a later stage of the infectious cycle that occurs within the infected cell. Antibodies against HA are described as ‘neutralising’ because they prevent virus infection.
By changing the HA protein, equine influenza can avoid recognition by these neutralising antibodies. NA is also involved in virus entry, it is thought to help break through the mucus layer that protects the respiratory tract. It also plays a part in virus release, enabling newly formed virus particles to escape from the surface of the cell that made them. Antibodies against NA are thought to block this process, preventing the virus from spreading to new cells. By changing the NA protein, the virus can avoid inhibition by these antibodies and go on to infect new cells.
Equine influenza virus belongs to a family of viruses that have RNA as their genetic material rather than DNA. RNA viruses tend to mutate more rapidly than DNA viruses. The virus has an enzyme called RNA-dependent RNA polymerase that is responsible for making new RNA copies of the virus genetic material for packaging into new virus particles. This is an essential step during the virus life cycle. Compared to the polymerase enzymes found in DNA viruses, the influenza polymerase makes more mistakes when it is copying the virus RNA and this is how changes are made in the genes that code for HA and NA.
As well as undergoing antigenic drift, influenza viruses including equine influenza virus can change their genes by a process called antigenic shift. This is a much bigger rapid change, brought about by the virus-swapping sections of its genome with another influenza virus. This process is called reassortment and is possible because the virus genome is made up from eight separate segments of RNA, each individually packaged in a set of proteins. If a horse is infected with two different equine influenza viruses at the same time, the eight segments from each virus can be mixed up, generating progeny viruses with new combinations of segments compared to the two parent viruses. This can lead to new combinations of HA and NA that haven’t been seen before, meaning there is no immunity to the new virus. This has happened during the evolution of human influenza viruses and resulted in the influenza pandemics of 1957, 1968 and 2009. In two of these examples, human influenza viruses swapped genes with avian viruses, leading to viruses that replicated well in humans but had a new HA gene from an avian virus.
In the 2009 pandemic, a new reassortant virus was generated in pigs then transmitted to humans. Reassortment has also happened with equine influenza viruses. The two different subtypes of equine influenza viruses, H7N7 and H3N8, underwent reassortment resulting in viruses that had most of the internal components of the H3N8 virus but with the HA and NA surface proteins from the H7N7 virus. Eventually these viruses died out and the only equine influenza viruses now in circulation are H3N8. There has been reassortment amongst the different sublineages of equine H3N8 viruses too, for example several of the viruses isolated in the UK during 2009 had a mixture of Florida clade 1 and Florida clade 2 HA and NA. Fortunately these reassortant viruses do not contain a novel HA or NA that has not been seen in horses before, so have not resulted in a major epidemic threat to horses.
In addition to antigenic drift and antigenic shift, the other source of potential new influenza viruses is an animal reservoir, such as birds. We know that horses can be infected by viruses belonging to the H3N8 and H7N7 subtypes and both of these are found in wild aquatic birds. It is thought that the 1963 H3N8 equine pandemic probably arose as a result of cross-species transmission from birds to horses in South America. Such an event happened in China in 1989, when an avian H3N8 infected horses with a much higher mortality rate than is usual for equine influenza. This virus spread amongst horses within China but died out after a relatively short time. It is possible that further avian-equine cross species transmission events could take place, however the virus must then adapt to its new host in order to become established in horses and be able to transmit efficiently from horse to horse. This will require mutations in various virus genes that help the virus attach to and replicate in cells lining the horse’s respiratory tract and spread via droplet infection to other horses.
TO READ MORE --
BUY THIS ISSUE IN PRINT OR DOWNLOAD -
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
HBLB Research on Injuries in Flat Racing: Nature versus Nurture
By Kristien Verheyen & Sarah Rosanowski
Musculoskeletal injuries are an inherent risk of horseracing, and they are the primary cause of thoroughbreds failing to train and race, or even retiring altogether. In addition to the evident equine welfare concerns, racehorse injuries also have economic consequences and impact on jockey safety. The industry remains committed to investigating causes of injury and associated risk factors, which can inform strategies aimed at minimising their occurrence. Advancements in methods of identification, management, and prevention of musculoskeletal disease and injury in Thoroughbreds and improved training and racing environments to enhance the safety, health, and wellbeing of racehorses have long been strategic priorities of the Horserace Betting Levy Board (HBLB)’s veterinary research funding program in Great Britain.
In 2014, the HBLB funded a research team at the Royal Veterinary College in London to undertake a detailed study of injuries and other veterinary events occurring in flat racehorses on race day. The purpose of the project was to establish causes of fatal and non-fatal injuries occurring in British flat racing and to examine associated risk factors. The research also set out to measure heritability of common injury types and conditions, and to investigate genetic and environmental correlations between injury and race performance.
The study team had access to detailed race and performance data from all Thoroughbreds racing on the flat in Great Britain over a 14-year study period from 2000 – 2013. These were then linked to veterinary reports of injury or conditions attended to by a veterinary surgeon on race day over the same time period, provided by the British Horseracing Authority (BHA). Finally, extensive pedigree data were added to enable investigation of heritability of race day injury and genetic correlations between injury types, and between injury and performance.
Descriptive findings
The final 14-year dataset included nearly 68,000 horses making over 800,000 starts in around 77,000 flat races. The majority of races -- 67% of them -- were run on the turf, with 33% of races taking place on all-weather tracks.
Just under 8,000 veterinary events were recorded over the study period, from which an incidence of nine events per 1000 starts was calculated. The most common incidents requiring veterinary attention on the racecourse were soft tissue injuries other than tendon and ligament injuries, e.g. wounds, lacerations, or muscle strains. Unspecified lameness and respiratory conditions were also common, accounting for around a fifth of veterinary reports each. Less than 10% of veterinary events had a fatal outcome, and the overall incidence of fatality was 0.8 per 1000 starts. Although bone injury was cited in only 14% of the veterinary reports overall, they accounted for the vast majority (77%) of the fatalities.
All-weather racing
Racing on all-weather tracks traditionally carries a higher risk of injury than racing on turf, which was reaffirmed in the current analyses. Therefore, the researchers also specifically investigated risk factors for fatality, distal limb fracture, and epistaxis (nose bleeds) in all-weather racing. These analyses were restricted to the ca. 258,000 all-weather starts in the dataset and included additionally collected information from the racecourse clerks on surface types and maintenance. The fatality incidence in all-weather racing was 0.9 per 1000 starts. Distal limb fracture occurred in around 1 in 1000 starts and epistaxis in 1.6 per 1000 starts. Risk factors varied for each outcome, although some factors were similar across outcomes including the going, racing intensity, horse age, age at first start, and horse and trainer performance variables. Generally, older horses and those that had started racing at an older age were at higher risk of an adverse outcome although for fatality, older horses that had started racing as two-year-olds were at highest risk. This association may be due to accumulation of microdamage in bone, which increases with increasing age as an effect of exercise accumulation over time and can ultimately lead to failure.
TO READ MORE --
Order this issue in print or download
Do the muscles of the respiratory system affect performance?
Over the last two decades the Horserace Betting Levy Board (HBLB) has funded substantial research to understand how various body systems respond to training. For example, because of this HBLB investment we now know that the hearts of thoroughbred racehorses get bigger as a response to athletic training and that big hearts are typically associated with better performers. We also know that bones respond to training by remodelling and hence become better prepared for the strains associated with galloping.
Streptococcus zooepidemicus - The bug that can place bets
The Horserace Betting Levy Board (HBLB) has invested over £7 million to protect racing and ensure horse welfare by disease surveillance and research on prevention of equine infections over the last decade. Infection with bacteria is one of the important causes. One bug in particular that can be found in many cases is Streptococcus zooepidemicus.
Respiratory disease affects a large proportion of young horses around the world, reducing performance with significant disruption to training and racing schedules. Inflammatory airway disease affects young horses in particular and it generally causes mucus in the trachea. Some estimates suggest that in British Flat racing yards, for every 100 horses, each month there will be nine cases.
Coughing and nasal discharge can last around eight weeks and some animals are affected again and again. All of which leads to significant cost to the racing industry. As a result, this problem has been a long-standing focus of attention for the Horserace Betting Levy Board’s (HBLB) veterinary research efforts.
Bacterial genetic code
One reason why bacteria from the same species might affect horses differently is that there are different strains within a bacterial species. This is rather like different breeds of horses – a Falabella pony is the same species as a thoroughbred – but it looks and acts very differently. All living things, from human to single-cell algae in the ocean, have a genetic code written in DNA. Understanding this genetic code can reveal how organisms live and function.
“In the same way as Strangles, a horse that has recovered from Streptococcus zooepidemicatus might no longer be outwardly affected itself but it may still carry the bug”
An HBLB-funded collaborative team working in Dr Andrew Waller’s lab at the Animal Health Trust and Professor Josh Slater’s lab at the Royal Veterinary College have set out to unlock the genetic make-up of different strains of Streptococcus zooepidemicus in order to understand better if some strains of this bacteria cause disease while others are relatively harmless. The researchers also looked at how different strains of Streptococcus interact with the horses’ immune system. The ulitmate goal of this research is to gain the knowledge which will lead to new vaccines.
A global research effort
The researchers started by developing a technique to produce a unique ‘genetic fingerprint’ with which to identify each different strain of the Streptococcus. They then tested samples from sick horses around the world and so far 318 different types of S. zooepidemicus have been identified with two particular strains being responsible for outbreaks of respiratory disease.
Insight from an ancient threat: Strangles
The researchers had some clues about what they might find in the samples from horses with Streptococcus zooepidemicus infection because they already had extensive experience in similar research in Strangles. Strangles is one of the oldest known, feared and most frequently reported infectious diseases of horses throughout the world. Typical signs of Strangles include abscessation of the lymph nodes in the head and neck, with swelling to such an extent that some horses are literally suffocated. It is caused by a relative of Streptococcus zooepidemicus, known as Streptotococcus equi. With Strangles, it is very clear that some recovered horses become carriers. Carriers show no outward signs and this hidden infection enables the bacteria to be spread around undetected.
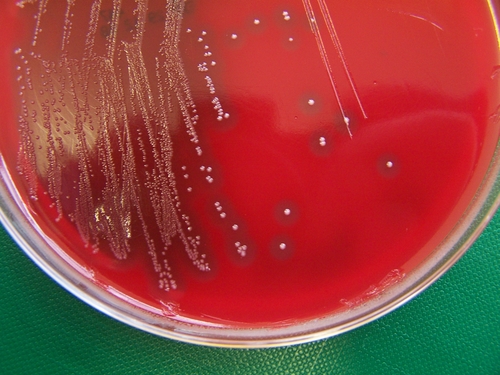
S. zooepidemicus colonies grown on a blood agar culture plate
Silent carriers lead to infection in youngsters
In the same way as Strangles, a horse that has recovered from Streptococcus zooepidemicatus might no longer be outwardly affected itself but it may still carry the bug. For example, the particular strain responsible for the outbreak of respiratory disease in Sweden was found in a healthy horse eight months after the horse made a full clinical recovery.
This persistence of S. zooepidemicus in thoroughbred racehorses that have recovered from respiratory disease allows transmission to susceptible animals and can occur when, for example, older recovered horses are mixed with the next year’s intake of young horses. It is likely that immunity to one strain of Streptococcus does not fully protect a horse from all the other strains, so young horses can often succumb to a succession of respiratory infections as they gradually build up immunity to mix of Streptococcus zooepidemicus strains that persist in that particular yard.
Bacterial balancing acts
In order to be able persist in recovered horses Streptococcus zooepidemicus must be able to survive despite the fact it is being attacked by the horse’s immune response, and at the same time, the bug must be ready to infect a susceptible animal should the opportunity arise. S. zooepidemicus strains have proteins on their surface and some of these proteins inactivate the horse’s immune response. Other proteins enable the bacteria to stick to the internal surfaces of the horse in order to establish the infection, almost like an ice climber clinging to the surface of a glacier with the crampons on his boots. If he loses his crampons, he is in big trouble.
Thus, these surface proteins play key roles for the bacteria, but they are also a vunerable point and can be targeted by the horse’s immune response to disable the bacteria. Therefore, balancing the array of surface proteins displayed with the particular requirements of the bacteria at any given time is critical if the bacteria are to successfully establish an infection and transmit to a new susceptible animal.
THERE'S MORE TO READ ONLINE....
THIS ARTICLE FIRST APPEARED IN EUROPEAN TRAINER - ISSUE 47
TO READ THIS ARTICLE IN FULL - CLICK HERE
Author: Celia Marr, Andrew S. Waller & Josh Slater
HBLB tendon & ligament research - equine injury prevention and management
Professor Celia M Marr looks into the work carried out by the HBLB over the past 50 years aimed at the prevention and management of tendon and ligament injuries, most recently looking at research into stem cells.